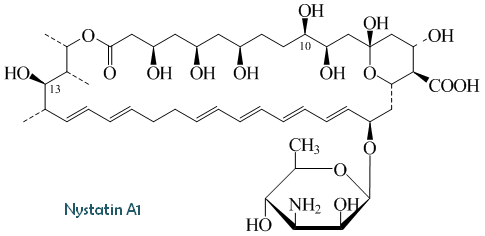
Nystatin : Cluster information
 Compound
Compound
| Entry name | Nystatin |
|---|---|
| PKS Type | TypeI modular |
| Starter Unit | acetyl-CoA |
| Chain Length | 19 |
| Sugar Unit | mycosamine |
| Classification | Polyene Macrolide |
| Activity | Antifungal |
| Composition | A1: C47H75NO17 |
 Original source
Original source
| Organism | Streptomyces noursei |
|---|---|
| Strain | ATCC 11455 (=NBRC 15452) |
| Contig | AF263912 |
 PKS/NRPS Module
PKS/NRPS Module
| Nysta_00130 nysA | 0 |  | acetyl-CoA |
| Nysta_00140 nysB | 1 |  | methylmalonyl-CoA |
| 2 |  | methylmalonyl-CoA not conserved YASHS(S->G) | |
| Nysta_00150 nysC | 3 |  | malonyl-CoA |
| 4 |  | malonyl-CoA | |
| 5 |  | malonyl-CoA | |
| 6 |  | malonyl-CoA | |
| 7 |  | malonyl-CoA | |
| 8 |  | malonyl-CoA | |
| Nysta_00050 nysI | 9 |  | malonyl-CoA |
| 10 |  | malonyl-CoA | |
| 11 |  | methylmalonyl-CoA | |
| 12 |  | malonyl-CoA | |
| 13 |  | malonyl-CoA | |
| 14 |  | malonyl-CoA | |
| Nysta_00060 nysJ | 15 |  | malonyl-CoA |
| 16 |  | malonyl-CoA | |
| 17 |  | malonyl-CoA | |
| Nysta_00070 nysK | 18 |  | malonyl-CoA |
 Reference
Reference
- Biosynthesis of the polyene antifungal antibiotic nystatin in Streptomyces noursei ATCC 11455: analysis of the gene cluster and deduction of the biosynthetic pathway.
- Brautaset T, Sekurova ON, Sletta H, Ellingsen TE, StrLm AR, Valla S, Zotchev SB[PMID: 10873841]Chem Biol. 7 (2000) 395-403
- In vivo analysis of the regulatory genes in the nystatin biosynthetic gene cluster of Streptomyces noursei ATCC 11455 reveals their differential control over antibiotic biosynthesis.
- Sekurova ON, Brautaset T, Sletta H, Borgos SE, Jakobsen M OM, Ellingsen TE, Strom AR, Valla S, Zotchev SB[PMID: 14973031]J Bacteriol. 186 (2004) 1345-54
- An unexpected role for the putative 4'-phosphopantetheinyl transferase-encoding gene nysF in the regulation of nystatin biosynthesis in Streptomyces noursei ATCC 11455.
- Volokhan O, Sletta H, Sekurova ON, Ellingsen TE, Zotchev SB[PMID: 15990252]FEMS Microbiol Lett. 249 (2005) 57-64
- Nystatin biosynthesis and transport: nysH and nysG genes encoding a putative ABC transporter system in Streptomyces noursei ATCC 11455 are required for efficient conversion of 10-deoxynystatin to nystatin.
- Sletta H, Borgos SE, Bruheim P, Sekurova ON, Grasdalen H, Aune R, Ellingsen TE, Zotchev SB[PMID: 16251298]Antimicrob Agents Chemother. 49 (2005) 4576-83
- Characterization of the P450 monooxygenase NysL, responsible for C-10 hydroxylation during biosynthesis of the polyene macrolide antibiotic nystatin in Streptomyces noursei.
- Volokhan O, Sletta H, Ellingsen TE, Zotchev SB[PMID: 16597951]Appl Environ Microbiol. 72 (2006) 2514-9
- Analysis of the mycosamine biosynthesis and attachment genes in the nystatin biosynthetic gene cluster of Streptomyces noursei ATCC 11455.
- Nedal A, Sletta H, Brautaset T, Borgos SE, Sekurova ON, Ellingsen TE, Zotchev SB[PMID: 17905880]Appl Environ Microbiol. 73 (2007) 7400-7
- The in vitro characterization of polyene glycosyltransferases AmphDI and NysDI.
- Zhang C, Moretti R, Jiang J, Thorson JS[PMID: 18798210]Chembiochem. 9 (2008) 2506-14
- New nystatin-related antifungal polyene macrolides with altered polyol region generated via biosynthetic engineering of Streptomyces noursei.
- Brautaset T, Sletta H, Degnes KF, Sekurova ON, Bakke I, Volokhan O, Andreassen T, Ellingsen TE, Zotchev SB[PMID: 21764946]Appl Environ Microbiol. 77 (2011) 6636-43
- Initiation of polyene macrolide biosynthesis: interplay between polyketide synthase domains and modules as revealed via domain swapping, mutagenesis, and heterologous complementation.
- Heia S, Borgos SE, Sletta H, Escudero L, Seco EM, Malpartida F, Ellingsen TE, Zotchev SB[PMID: 21821762]Appl Environ Microbiol. 77 (2011) 6982-90
- In vivo investigation of the substrate recognition capability and activity affecting amino acid residues of glycosyltransferase FscMI in the biosynthesis of candicidin.
- Lei X, Kong L, Zhang C, Liu Q, Yao F, Zhang W, Deng Z, You D[PMID: 23324745]Mol Biosyst. 9 (2013) 422-30
- Stereochemistry of reductions catalyzed by methyl-epimerizing ketoreductase domains of polyketide synthases.
- You YO, Khosla C, Cane DE[PMID: 23659177]J Am Chem Soc. 135 (2013) 7406-9
- Coupled methyl group epimerization and reduction by polyketide synthase ketoreductase domains. Ketoreductase-catalyzed equilibrium isotope exchange.
- Garg A, Khosla C, Cane DE[PMID: 24161343]J Am Chem Soc. 135 (2013) 16324-7
 Data download
Data download
 History
History
- 2014-06-25[Update]
- 2013-12-27[Update]
- 2013-09-04[Update]
- 2013-01-16[Update]
- 2012-10-02[Update]
- 2012-03-28[Release]






