Risk Assessment on chemicals-For Better Understanding-5
Methods and Threshold Levels for Carcinogenicity Assessment
What is NOAEL (No Observed Adverse Effect Level)?
Unlike other non-cancerous symptoms, when a carcinogen infiltrates a gene to create cancer cells, there is no minimum level of carcinogen without the possibility of causing cancer, even the lowest level of carcinogen is still considered to have a potential of causing cancer.
The term “threshold” is used to indicate the amount of intake or the exposure of a chemical without a potential of adverse effects.
If a chemical has the potential of exhibiting adverse effects unless the exposure level is zero (none), this chemical is considered as having “no threshold.” Conversely, if a chemical has a minimum effective exposure level that does not exhibit adverse effects, the chemical is considered to have “a threshold”.
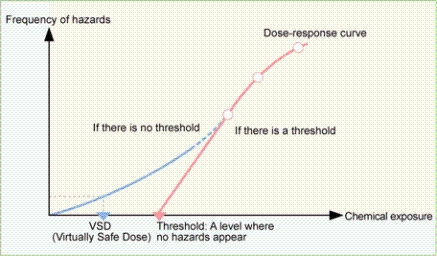
If there is no threshold of hazards, there is no NOAEL (No Observed Adverse Effect Level) and also the TDI (Tolerable Daily Intake)*1, therefore the method for Risk Assessment differs from the method used for cases where there is NOAEL.
*1: See “NOAEL (No Observed Adverse Effect Level) and TDI (Tolerable Daily Intake)”
One method of Risk Assessment used in such cases adopts “the amount causing carcinogenesis at a probability of 1/100,000” as VSD (Virtually Safe Dose), instead of adopting NOAEL or TDI.
Carcinogenicity through injury of genes and mutagenicity etc. through activity on germ cells are now considered to have no threshold. *2
*2: There are multiple opinions regarding threshold for carcinogenicity. This topic is still controversial.
column
*”Initial Risk Assessment Report” under the NEDO Project evaluates carcinogenicity as shown on the below website.
Initial Risk Assessment Report
Carcinogens without gene injury (with threshold)
For these carcinogens, Risk Assessment with MOE (Margin Of Exposure) is carried out, similar to the method used for other types of toxicity.
The two factors, listed below, are additionally taken into account as uncertainty factors (UF).
- Carcinogenicity (x1 to 10)
- Corresponding to seriousness related to cancer cell type, favorite site, expression etc. (x1 to 10)
Carcinogens with gene injury (no threshold)
Because there is no threshold, risk assessment by MOE (Margin Of Exposure) is not possible. So, if quantitative evaluation by evaluation organizations such as EPA (Environment Protection Agency, USA) and WHO (World Health Organization) can be utilized, the unit risk etc. expressing the probability of carcinogenicity* is shown.
However, when final judgment is made, unit risk or the like serves only as reference information and is treated as “a candidate of substances needed for detailed Risk Assessment.”
Footnote
- *Unit risk:
- The upper limit of the predicted risk for carcinogenesis following daily exposure to a chemical at a concentration of 1 µg/L (water) or 1 µg/m3 (air) throughout one’s lifetime (70 years).
Contact us
- Chemical Management Center, National Institute of Technology and Evaluation
-
Phone number:+81-3-3481-1977
Fax number:+81-3-3481-2900
Address:2-49-10 Nishihara, Shibuya-ku, Tokyo 1510066, Japan MAP
Contact Form