Risk Assessment on chemicals-For Better Understanding-7
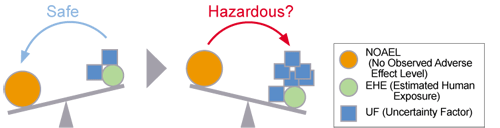
UF (Uncertainty Factor)
Various data used for risk assessment involve many uncertain elements.
(For example, it is impossible to prove that the carcinogenicity of a chemical in humans appears at a probability of 1 in a million, by conducting a test involving the exposure of 1 million human subjects to the chemical for the rest of their lives. For this reason, experiments using rats and other experimental animals are carried out instead of humans. The carcinogenicity of a chemical in humans, estimated on the basis of the data from these, toxicity tests, involved uncertainties.)
In risk assessment, UF (Uncertainty Factor) is set to enable risk assessment while avoiding underestimation of the risk due to uncertainties so that risk assessment can be done with a sufficient safety margin.
Generally, UF is initially set at 100, with interspecies variation (x10, difference between animal and humans) and intraspecies variation (x10) taken into account.
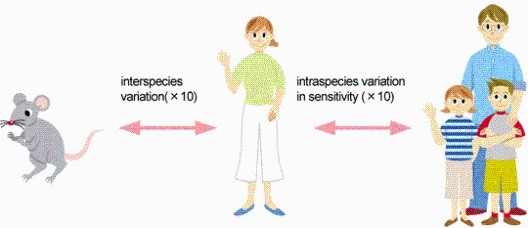
UF is supplemented if there is any uncertainty related to the study period, reliability and other features of the toxicity tests.
UF is basically 1 or 10 but can assume other values depending on the degree of uncertainty. (At present, there are no global rules about UF. Individual countries and evaluation organizations select a value of UF deemed as appropriate.)
When multiple factors are taken into consideration, the factors are multiplied by each other to yield UFs (product of Uncertainty Factors).
As this value gets higher, the Risk Assessment becomes less reliable.
If this value is excessively high, it is possible that “no risk” is judged to be “a risk” because of low reliability of the data used as the rationale. So, it is essential to carefully interpret the results of risk assessment.
Terms such as coefficient of explanation, evaluation coefficient, correction coefficient and safety coefficient are sometimes used with the same meaning as UF (Uncertainty Factor).
column
*”Initial Risk Assessment Report” under the NEDO Project shows UFs (product of Uncertainty Factors) on the following website.
Initial Risk Assessment Report
UFs (product of Uncertainty Factors) = (interspecies variation) x (intraspecies variation) x (use of LOAEL (Lowest Observed Adverse Effect Level) x (test period) x (correction coefficient)
UF for each factor is shown below.
| Interspecies variation | 10 (based on toxicity test data), 1 (based on human data) |
|---|---|
| Intraspecies variation | 10 |
| The use of LOAEL (Lowest Observed Adverse Effect Level) | 10 (if LOAEL is converted into NOAEL), 1 (if NOAEL is used) |
| Test period (if short-term test data is used) | 10 (1-month test), 5 (3-month test), 2 (6-month test), 1 (6-month or longer test) |
| Correction coefficient | A coefficient added at the expert’s judgment, depending on the type, quality, etc. of the test. If there is no addition, correction coefficient is set at 1. This coefficient involves the following viewpoints, for example:
|
Footnote
*GLP (Good Laboratory Practice): GLP system is aimed at ensuring reliability of test results through checking the operational management, testing facilities, test plans, internal audit system, reliability guarantee system, test results and so on. Every three years, confirmation and renewal is necessary.
Details are given on the page on the GLP System of the NITE website.
Contact us
- Chemical Management Center, National Institute of Technology and Evaluation
-
Phone number:+81-3-3481-1977
Fax number:+81-3-3481-2900
Address:2-49-10 Nishihara, Shibuya-ku, Tokyo 1510066, Japan MAP
Contact Form