Monen_00090 : CDS information
 Location
Location
| Organism | Streptomyces cinnamonensis |
|---|---|
| Strain | ATCC 15413 |
| Entry name | Monensin |
| Contig | AF440781 |
| Start / Stop / Direction | 8,531 / 6,045 / - [in whole cluster] 8,531 / 6,045 / - [in contig] |
| Location | complement(6045..8531) [in whole cluster] complement(6045..8531) [in contig] |
| Type | CDS |
| Length | 2,487 bp (828 aa) |
 Annotation
Annotation
| Category | 1.1 PKS |
|---|---|
| Product | polyketide synthase |
| Product (GenBank) | ketosynthase-like protein |
| Gene | ksX |
| Gene (GenBank) | |
| EC number | |
| Keyword |
|
| Note |
|
| Note (GenBank) |
|
| Reference |
|
| Related Reference |
|
 PKS/NRPS Module
PKS/NRPS Module
| 13 | 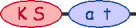 |
 Sequence
Sequence
>polyketide synthase [ketosynthase-like protein] MANEEKLVEYLKWTTAELHQAQQQLRELKAAQHEPIAVVSMACRLPGKTRTPDDLWDLVS EGRDAVTGFPDDRAWELPEERPYAELGGFLDDAAGFDAGFFDISDTEAVATEPLQRLMLH LAWETVERGHIAPHTLRSTLTGVYVGATGHDYATRLETAPDELLPYLGGGTSGSLVSGRI AYALGLEGPAISVDTACSSSLVALHLACQALRRGECGLALAGGGTVMSTPHTFHAFAHQK SLAQDGRCKPFAAAADGMGLGEGVGLVLLERLGDARKNGHPVLAVIRGSAVNQDGAGYGL AAPNGPSQQHVIRAALADAGLTPDQIDAVEAHGTGTPIGDAIEVQALLATYGADRSPDRP LWLGSVKSNTGHTQGAAGAAALIKMVQAFRHGTLPPTLHVDRPTPLAAWKKGAVRLLTEA VDWPRREEPRRVGISAFATSGTNAHLILEEPPVDEAPVPDAARDQTSPVAPELPVAWSLS ARTPEALRAQAKALVTHLAATDPAPSPAEVAYSLAATRSPLEHRAVLTGTDHTELLAAAR ALAAGEDHPDLVRSTPGAGPKKIAWHFDGRPADGVTTGAAPGAKPGATFGATFGAAFGGA EFHSAFPLFASAFDEARALLDTHLPTPLPTPHSELARFAVHTALARLLLETGVRPHTLTG DGVGHIAAAYAAGILTLDDACRLAAAHAAAAQAAEGEQPAPPDAYEPVLKQLTFQRATLT LTSTAPADTPIASADYWHHHLTSPAPTAPPTPETHTLLHLGALSPEGTQTSAVSALLTAL ARLHTTGGTVDWTPLVRRTPHPRTIDLPTYSFQATRYWLHDHTAHAAV
>polyketide synthase [ketosynthase-like protein] GTGGCGAACGAAGAGAAGCTCGTCGAATACCTCAAGTGGACGACGGCCGAGCTCCACCAG GCCCAGCAGCAGCTGCGCGAACTGAAGGCCGCACAGCACGAGCCGATCGCCGTCGTCTCC ATGGCCTGCCGGCTGCCCGGCAAGACCCGCACCCCGGACGACCTGTGGGATCTGGTGTCC GAGGGCCGCGACGCCGTCACCGGCTTCCCCGACGACCGCGCCTGGGAACTCCCCGAGGAA CGCCCGTACGCGGAGCTCGGCGGGTTCCTGGACGACGCGGCCGGCTTCGACGCGGGCTTC TTCGACATCAGCGACACCGAGGCCGTGGCCACCGAACCCCTCCAGCGCCTCATGCTCCAC CTCGCGTGGGAGACCGTCGAACGCGGCCACATCGCCCCGCACACCCTGCGCTCCACCCTC ACCGGCGTCTACGTCGGCGCCACCGGGCACGACTACGCGACACGGCTCGAGACCGCGCCC GACGAGCTGCTGCCCTATCTGGGCGGCGGCACGTCCGGCAGCCTCGTCTCCGGCCGCATC GCCTACGCCCTCGGCCTCGAGGGCCCCGCCATCAGCGTGGACACGGCCTGCTCGTCCTCC CTGGTCGCCCTCCACCTGGCCTGCCAGGCGCTGCGCCGCGGGGAGTGCGGCCTCGCCCTC GCCGGCGGCGGCACCGTCATGTCGACGCCGCACACCTTCCACGCCTTCGCCCACCAGAAG TCGCTCGCGCAGGACGGCCGTTGCAAACCGTTCGCCGCCGCGGCCGACGGCATGGGCCTC GGTGAAGGCGTCGGCCTCGTCCTGCTTGAGCGGCTCGGCGACGCCAGGAAGAACGGCCAC CCGGTGCTCGCCGTCATCCGCGGCTCCGCGGTCAACCAGGACGGCGCCGGATACGGCCTC GCCGCCCCCAACGGCCCCTCCCAGCAGCATGTGATCCGCGCCGCCCTCGCCGACGCCGGG CTCACCCCGGACCAGATCGACGCCGTCGAGGCGCACGGGACGGGCACCCCCATCGGCGAC GCCATCGAGGTCCAGGCCCTCCTCGCCACCTACGGCGCCGACCGCTCCCCCGACCGGCCC CTGTGGCTCGGCTCCGTCAAGTCCAACACGGGGCACACGCAGGGGGCCGCGGGTGCGGCC GCGCTCATCAAGATGGTCCAGGCGTTCCGGCACGGCACCCTGCCGCCGACCCTCCACGTC GACCGCCCGACGCCCCTCGCCGCCTGGAAGAAGGGCGCGGTACGGCTGCTCACCGAGGCG GTCGACTGGCCCCGCCGCGAGGAGCCCCGCCGGGTCGGCATCTCCGCCTTCGCCACGTCC GGCACGAACGCGCACCTCATCCTCGAAGAGCCGCCGGTGGACGAGGCTCCGGTGCCGGAT GCCGCCCGCGACCAGACGTCGCCCGTTGCCCCGGAACTCCCGGTGGCCTGGAGCCTGTCC GCTCGTACACCCGAGGCCCTGCGGGCACAGGCGAAGGCCCTCGTCACCCACCTGGCGGCC ACCGACCCCGCGCCCTCCCCCGCCGAGGTCGCCTACTCGCTCGCCGCCACCCGCAGCCCC CTGGAACACCGCGCCGTCCTCACCGGCACCGACCACACCGAACTCCTCGCCGCCGCCCGC GCCCTGGCCGCCGGAGAGGACCACCCGGACCTGGTCAGGTCCACGCCCGGGGCCGGCCCG AAGAAGATCGCCTGGCACTTCGACGGCCGCCCGGCCGACGGGGTGACGACGGGGGCGGCA CCCGGAGCCAAACCCGGCGCGACATTCGGCGCTACATTCGGCGCGGCTTTCGGAGGTGCC GAGTTCCACTCGGCGTTCCCGCTCTTCGCGTCCGCCTTCGACGAAGCGCGCGCGCTCCTC GACACCCATCTGCCGACTCCCCTCCCCACTCCCCACTCCGAACTGGCGCGCTTCGCGGTC CACACCGCGCTCGCGCGGCTGCTCCTGGAAACGGGGGTACGCCCCCACACCCTCACCGGC GACGGTGTCGGCCACATCGCCGCCGCGTACGCCGCGGGAATCCTGACCCTCGACGACGCG TGCCGCCTGGCCGCCGCCCACGCCGCTGCCGCCCAGGCCGCCGAGGGTGAACAACCGGCT CCGCCCGACGCCTACGAGCCCGTGCTGAAGCAGCTGACGTTCCAACGCGCCACGCTCACG CTCACCAGCACTGCCCCGGCCGACACCCCCATCGCCTCCGCCGACTACTGGCACCACCAC CTCACCTCGCCGGCCCCCACCGCACCCCCCACCCCCGAGACCCACACGCTTCTCCACCTG GGCGCGCTCTCACCGGAAGGCACGCAAACCTCCGCCGTAAGCGCCCTGTTGACCGCCCTC GCGCGGCTTCACACCACGGGCGGCACCGTCGACTGGACCCCTCTCGTCCGGCGCACCCCC CACCCCCGGACCATCGACCTCCCGACGTACTCCTTCCAGGCCACGCGTTACTGGCTGCAC GACCACACCGCGCACGCCGCGGTGTGA
 Feature
Feature
| BLASTP Database:UniProtKB:2011_09 |
|
|---|---|
| InterPro Database:interpro:38.0 |
IPR001227 Acyl transferase domain (Domain)
IPR014030 Beta-ketoacyl synthase, N-terminal (Domain)
IPR014031 Beta-ketoacyl synthase, C-terminal (Domain)
IPR014043 Acyl transferase (Domain)
IPR015083 Polyketide synthase, docking (Domain)
IPR016035 Acyl transferase/acyl hydrolase/lysophospholipase (Domain)
IPR016038 Thiolase-like, subgroup (Domain)
IPR016039 Thiolase-like (Domain)
IPR018201 Beta-ketoacyl synthase, active site (Active_site)
IPR020841 Polyketide synthase, beta-ketoacyl synthase domain (Domain)
|
| SignalP | No significant hit |
| TMHMM | No significant hit |


 Monen_00080
Monen_00080


