Amphotericin B : Cluster information
 Compound
Compound
| Entry name | Amphotericin B |
|---|---|
| PKS Type | TypeI modular |
| Starter Unit | malonyl-CoA |
| Chain Length | 19 |
| Sugar Unit | mycosamine |
| Classification | Polyene Macrolide |
| Activity | Antifungal |
| Composition | C47H73NO17 |

 Original source
Original source
| Organism | Streptomyces nodosus |
|---|---|
| Strain | ATCC 14899 (=NBRC 12895) |
| Contig | AF357202 |
 PKS/NRPS Module
PKS/NRPS Module
| Amph_00150 amphA | 0 | 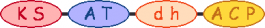 | acetyl-CoA |
| Amph_00160 amphB | 1 | 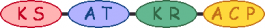 | methylmalonyl-CoA |
| 2 |  | methylmalonyl-CoA not conserved YASHS(S->G) | |
| Amph_00170 amphC | 3 |  | malonyl-CoA |
| 4 |  | malonyl-CoA | |
| 5 |  | malonyl-CoA | |
| 6 |  | malonyl-CoA | |
| 7 |  | malonyl-CoA | |
| 8 |  | malonyl-CoA | |
| Amph_00040 amphI | 9 |  | malonyl-CoA |
| 10 |  | malonyl-CoA | |
| 11 |  | methylmalonyl-CoA | |
| 12 |  | malonyl-CoA | |
| 13 | 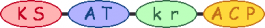 | malonyl-CoA | |
| 14 |  | malonyl-CoA | |
| Amph_00050 amphJ | 15 |  | malonyl-CoA |
| 16 |  | malonyl-CoA | |
| 17 |  | malonyl-CoA | |
| Amph_00060 amphK | 18 |  | malonyl-CoA |
 Reference
Reference
- Amphotericin biosynthesis in Streptomyces nodosus: deductions from analysis of polyketide synthase and late genes.
- Caffrey P, Lynch S, Flood E, Finnan S, Oliynyk M[PMID: 11451671]Chem Biol. 8 (2001) 713-23
- Biosynthesis of deoxyamphotericins and deoxyamphoteronolides by engineered strains of Streptomyces nodosus.
- Byrne B, Carmody M, Gibson E, Rawlings B, Caffrey P[PMID: 14700629]Chem Biol. 10 (2003) 1215-24
- Analysis and manipulation of amphotericin biosynthetic genes by means of modified phage KC515 transduction techniques.
- Carmody M, Byrne B, Murphy B, Breen C, Lynch S, Flood E, Finnan S, Caffrey P[PMID: 15563836]Gene. 343 (2004) 107-15
- Biosynthesis of amphotericin derivatives lacking exocyclic carboxyl groups.
- Carmody M, Murphy B, Byrne B, Power P, Rai D, Rawlings B, Caffrey P[PMID: 16079135]J Biol Chem. 280 (2005) 34420-6
- The in vitro characterization of polyene glycosyltransferases AmphDI and NysDI.
- Zhang C, Moretti R, Jiang J, Thorson JS[PMID: 18798210]Chembiochem. 9 (2008) 2506-14
- Redesign of polyene macrolide glycosylation: engineered biosynthesis of 19-(O)-perosaminyl-amphoteronolide B.
- Hutchinson E, Murphy B, Dunne T, Breen C, Rawlings B, Caffrey P[PMID: 20189107]Chem Biol. 17 (2010) 174-82
- Structural and functional analysis of A-type ketoreductases from the amphotericin modular polyketide synthase.
- Zheng J, Taylor CA, Piasecki SK, Keatinge-Clay AT[PMID: 20696392]Structure. 18 (2010) 913-22
- In vivo investigation of the substrate recognition capability and activity affecting amino acid residues of glycosyltransferase FscMI in the biosynthesis of candicidin.
- Lei X, Kong L, Zhang C, Liu Q, Yao F, Zhang W, Deng Z, You D[PMID: 23324745]Mol Biosyst. 9 (2013) 422-30
- Molecular dynamics studies of modular polyketide synthase ketoreductase stereospecificity.
- Mugnai ML, Shi Y, Keatinge-Clay AT, Elber R[PMID: 25835227]Biochemistry. 54 (2015) 2346-59
- Exploiting the genome sequence of Streptomyces nodosus for enhanced antibiotic production.
- Sweeney P, Murphy CD, Caffrey P[PMID: 26497174]Appl Microbiol Biotechnol. (2015)
- Substrate structure-activity relationships guide rational engineering of modular polyketide synthase ketoreductases.
- Bailey CB, Pasman ME, Keatinge-Clay AT[PMID: 26568113]Chem Commun (Camb). 52 (2016) 792-5
- Alteration of Polyketide Stereochemistry from anti to syn by a Ketoreductase Domain Exchange in a Type I Modular Polyketide Synthase Subunit.
- Eng CH, Yuzawa S, Wang G, Baidoo EE, Katz L, Keasling JD[PMID: 26976746]Biochemistry. 55 (2016) 1677-80
 Data download
Data download
 History
History
- 2016-12-27[Update]
- 2016-01-27[Update]
- 2015-02-12[Update]
- 2013-01-16[Update]
- 2012-10-02[Update]
- 2012-03-28[Release]






