Notification of acquisition of the genetic resources in Japan
Pursuant to Chapter 5 of the ABS Guidelines, NITE issues the documents concerning the acquisition of genetic resources in Japan called the “Notification of Acquisition of the Genetic Resources in Japan (‘Notification’)” NITE is authorized by the Ministry of Economy, Trade and Industry as the organization that issues the Notification.
What is the Notification useful for?
The Notification can show that genetic resources have been acquired in Japan. It is useful when showing of the provenance of the genetic resources is required, for example, export or utilization of the genetic resources in another country. Having the Notification may make various processes faster and easier.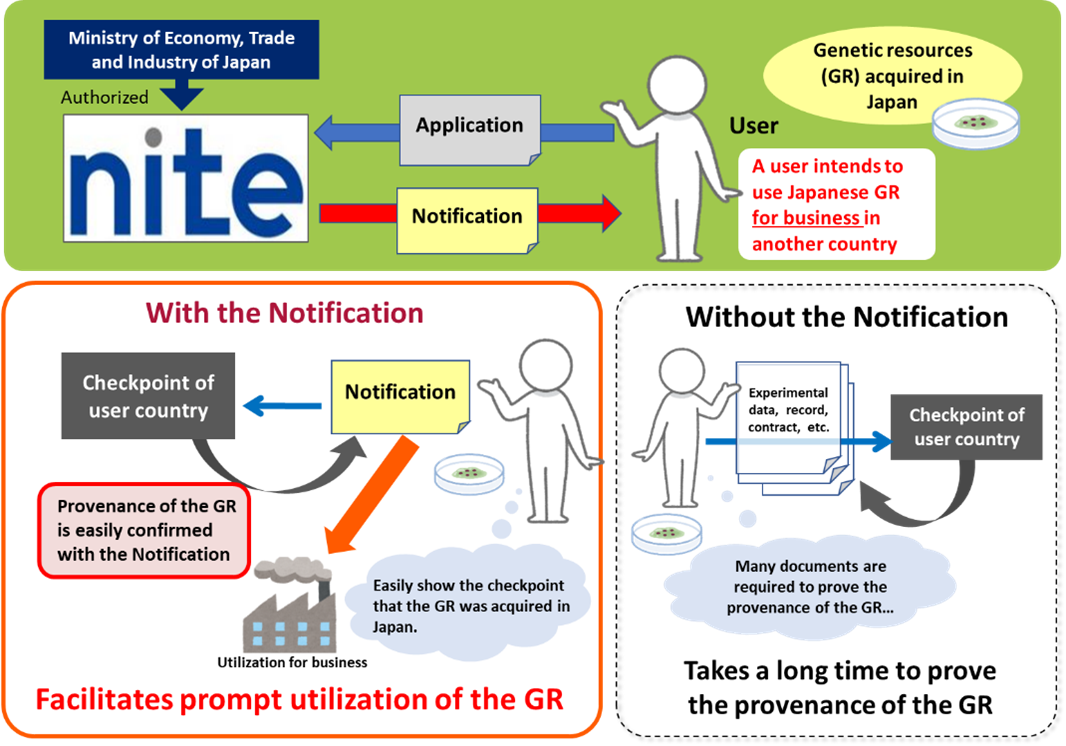
Sample Notification

<Please click to expand the image>
Conditions for issuance
All of the following conditions must be met for the issuance of the Notification.- 1. The “country of origin of the genetic resources” is Japan.
- *"Country of origin of genetic resources" means the country which possesses those genetic resources in in-situ conditions.
- *"In-situ conditions" means conditions where genetic resources exist within ecosystems and natural habitats, and in the case of domesticated or cultivated species, in the surroundings where they have developed their distinctive properties.
- 2. The “country providing the genetic resource" is Japan.
- *"Country providing genetic resources' means the country supplying genetic resources collected from in-situ sources, including populations of both wild and domesticated species, or taken from ex-situ sources, which may or may not have originated in that country.
- 3. The genetic resource is utilized for business under the jurisdiction of the Minister of Economy, Trade and Industry of Japan.
- 4. The genetic resource is NOT utilized under “The International Treaty on Plant Genetic Resources for Food and Agriculture” (ITPGRFA).
- 5. The genetic resource is NOT utilized under “The Pandemic Influenza Preparedness Framework” (PIPF).
Please refer to the Application Manual for the detailed application procedure.
Fees and Forms
| Process | Forms | Examples | Instructions | Fee* |
|---|---|---|---|---|
| New issuance | Application [word 49KB] Agreement [word 51KB] |
Application [PDF 235KB] Agreement [PDF 207KB] |
Application [PDF 145KB] Agreement [PDF 108KB] |
JPY 20,700 |
| Re-issuance | Application [word 49KB] |
Application Manual 【PDF 576KB】 |
Application Manual 【PDF 576KB】 |
JPY 6,000 |
| Revision | Application [word 49KB] Application for Revision |
JPY 6,000 | ||
| Change of Intent for Posting on Website |
Application [word 48KB] |
No fee | ||
| Invalidation | Application [word 48KB] |
No fee |
*Fees are paid for per application
List of the issued Notifications
The list of the “Notification of acquisition of the genetic resources” issued is available here.
Related Links
- [Ministry of Economy, Trade and Industry] About CBD
- [Ministry of the Environment] ABS national website of Japan
- [ABS Clearing-House] Legislative, Administrative or Policy Measure (MSR)
Contact us
- CBD Administration Division, Biological Resource Center, National Institute of Technology and Evaluation
-
Phone number:+81-3-3481-1963
Address:2-49-10 Nishihara, Shibuya-ku, Tokyo 1510066, Japan MAP
Contact Form


