NITE Alert_73
December 13, 2006
Alert -Desk Pad may pose Skin Lesions Hazard
The National Institute of Technology and Evaluation (NITE) has conducted investigations on desk pads in response to a report from a medical institution indicating that a patient was diagnosed with “allergic contact dermatitis* from using a desk pad". Results showed the causative substances were organic antimicrobials (2,3,5,6-Tetrachloro-4- [Methylsulfonyl] Pyridine) used for the desk pad, and the continuous contact with these substances caused allergic contact dermatitis.
NITE received reports of 13 similar incidents from medical institutions from August 2005 to July 2006. This alert has been issued prevent further incidents involving this product.
- *Note: Allergic contact dermatitis
Allergic contact dermatitis is caused by the body coming into contact with a causative agent that is absorbed through the skin. In some cases, depending on the individual, the causative agent is perceived as a foreign substance when it is absorbed, and the body prepares to expel the substance (sensitization). Once sensitized to the substance, any subsequent exposure will cause the body to overreact and produce a rash. Allergic contact dermatitis is not always provoked in everyone since it is subject to exposure time, one's physical conditions and skin sensitivity.
Alert:
Anyone who has this desk pad should discontinue use or take appropriate actions by referring to the company announcement.
1. Outline of the incident
A consumer developed dermatitis on both forearms while using a desk pad made of vinyl chloride resin (See image). As part of a treatment, a patch test was applied to the patient by the dermatologist. The patient tested positive with the relevant desk pad and was thus diagnosed with allergic contact dermatitis caused by the desk pad.
product sample
skin lesions caused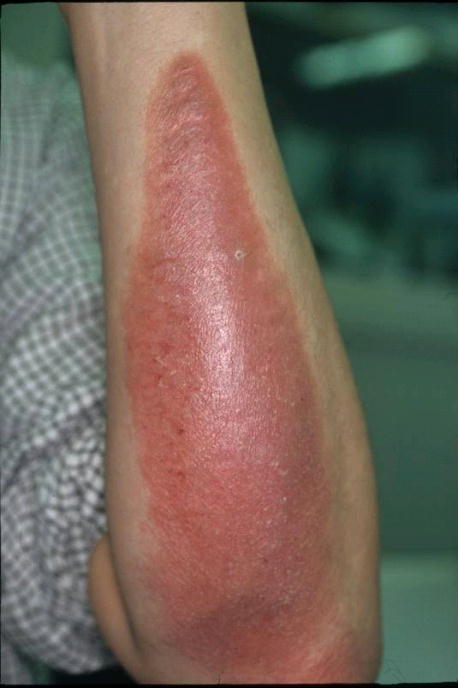
2. Investigation Summary
NITE has conducted investigations with the assistance of medical institutes to identify the causative substances. Chemical materials were extracted from the relevant product with organic solvents, and then analyzed through methods such as GC-MS after separation and purification in a silica gel column chromatogram process. Following results, we have identified organic antimicrobials (2,3,5,6-Tetrachloro-4- [Methylsulfonyl] Pyridine) as the causative substances.
- (Reference)
- Product name: Antibacterial and Nontransferable Soft Desk Pads
Images of the sample model number label
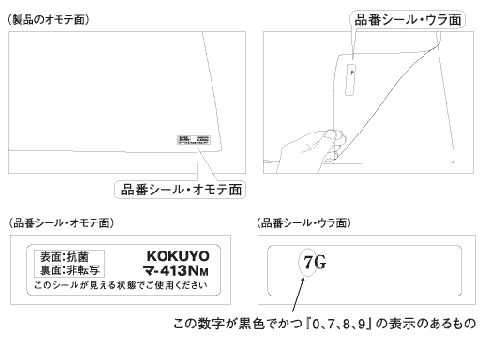
- Subject model numbers:
- Model numbers are indicated in black in a combination with one of the following letters and one of the following numbers 7, 8, 9 or 0. The label with the model number is located on the lower right of the product.
- Letters:
- A, E, G, H, I, M, N, O, R, S, T or Y
- Serial number:
- MA-400NM, MA-406NM, MA-407NM, MA-411NM, MA-412NM,
MA-413NM, MA-415NM, MA-416NM, MA4-17NM, MA-427NM,
MA-428NM, MA-447NM, MA-448NM, MA-467NM, MA-468NM,
MA-500N, MA-506N, MA-507N, MA-511N, MA-512N, MA-513N, MA-515N,
MA-516N, MA-517N, MA-527N, MA-528N, MA-547N, MA-548N,
MA-567N, MA-568N, MA-MX517N, MA-MX527N, MA-MX547N,
MA-MX567N (*MA: as in Japanese characters)- Note: Although the same serial numbers are used, products manufactured after September 2000 use different antibacterial agents, and consequently have no bearing in this matter.
- Kokuyo S & T Co., Ltd.:
- Kokuyo customer center 0120-201-594(toll-free)
http://www.kokuyo.co.jp/info/20061011.html
Fore more information;
National Institute of Technology and Evaluation (NITE)
Product Safety Technology Center, Product Safety Investigation Division
Contact us
- Consumer Product Safety Public Relations Division Product Safety Technology Center National Institute of Technology and Evaluation
-
Phone number:+81-6-6612-2066
Fax number:+81-6-6612-1617
Address:1-22-16 Nankokita, Suminoe-ku, Osaka-shi, Osaka 5590034, Japan MAP